All About Over the Counter Medicines from Regulation To Impact Of The Covid-19 Pandemic
Along with the Over the Counter Medicines, the consumer healthcare sector has witnessed a growing demand over the past few years, which has skyrocketed with the advent of the pandemic. The pandemic has led to a gradual increase in healthcare costs, while pressure on healthcare staff has increased. These developments have prompted more people to take their health into their own hands. This is the reason why we see an increased proportion of the population turning towards preventative care and non-prescription treatment for cases of minor illnesses.
The global OTC Drugs market is growing faster than what was previously estimated. The market will grow up to $157 billion in 2021 and reach up to $233.6 billion in 2028 at a CAGR of 5.8%. In 2020, the North American market for OTC products was $58.4 billion.
What are Over the Counter Medicines?
These are pharmaceutical products deemed safe for use without requiring a prescription from a healthcare giver. Such medicines are available in pharmacies and medical stores. These products can be sold legally without the requirement of a prescription. Even some groceries are stocked with such products.
Therapeutic Areas Covered By OTC Treatments?
These medicines treat some common symptoms such as cough, cold, allergy, flu, and body pain. Other health issues that can be treated with OTC medicines are heartburn and acne. They are also used for treating digestive and intestinal ailments.
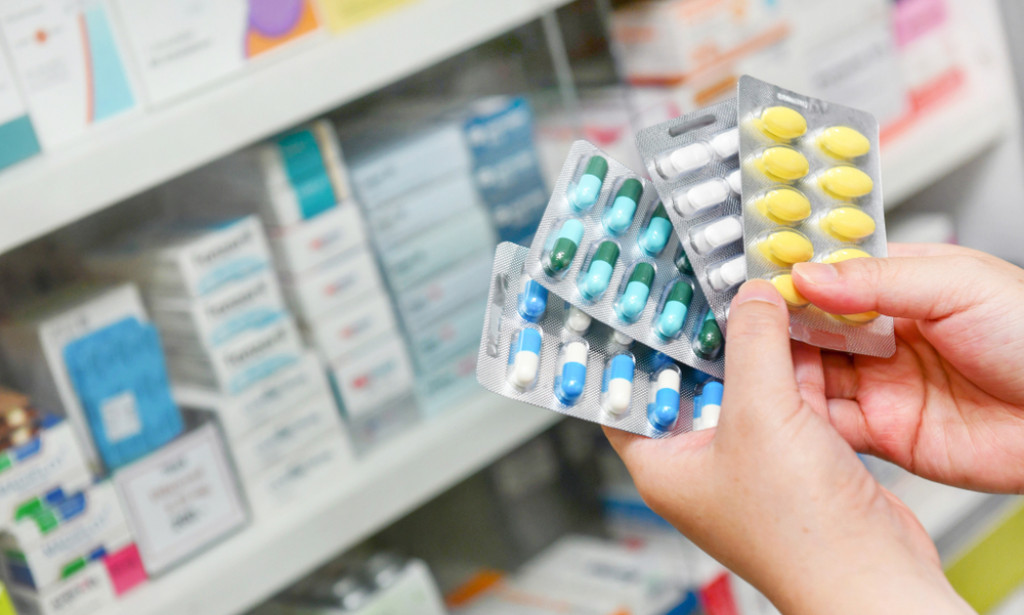
OTC medicines are available in different forms ranging from tablets to capsules to lozenges and oral liquids. They also come in the form of topical ointment and patches.
Regulations Surrounding OTC Medicines
Most countries, including the U.S., have stringent regulations in place to ensure that the Over the Counter medicines contain safe ingredients with effective uses to be sold without a prescription.
There may be some differences in the different OTC products being sold. While some classes are available at stores, others may only be found in pharmacies. In case of OTC products being sold at pharmacies, they have to be handed over by a registered pharmacist. In such instances, the pharmacies may ask the consumer a few questions to determine the situation and issue-specific information about the product in case it is being sold. Such sales by pharmacies may require an ID for approving the sale of a product. This requirement may also be to fulfill any purchase quantity restrictions.
In the U.S., OTC products are overseen by the Food and Drug Regulation Authority (FDA). Most of the FDA policies are monograph-driven. OTC monographs serve as recipe books that lay down the different conditions for the sale of these OTC products, such as active ingredients, doses, usages, product labeling, testing, and routes of administration.
Though the monograph process is still under reform in the U.S., there are still more than 80 therapeutic category monographs. There are different monographs for different classes of OTC products such as analgesics, antacids, and antihistamines.
In the U.S., an act known as the Cares Act was passed and signed into law in 2020. This act contains legislation intended to modernize the procedure through which OTC drugs would be regulated.
In the European Union, prescription drugs are evaluated by the European Medicines Agency, while the OTC drugs are reviewed at the national level. Germany, which is one of the largest national markets for OTC medicines, regulates these non-prescription medicines through its Federal Ministry of health.
Another large market is China, where the OTC medicines are regulated by the National Medical Products Administration (NMPA).
Impact of the Pandemic On Over The Counter Medicines
The pandemic has impacted all businesses and industries since it reared its ugly head. However, the noteworthy point is that the healthcare industry was one sector that witnessed tremendous changes. It oversaw an exponential growth during this time, with demand for some categories of OTC drugs and medicines growing by a large margin.
As the pandemic swept across the world, consumers started looking for ways to remaining healthy and boosting their immunity. The fear of an imminent illness in case of contracting the coronavirus fueled this mindset. They felt that consuming various vitamin supplements could help achieve these goals. The pandemic also contributed to a greater resolve to prevent illnesses like common flu and cold. However, at the same time, the public tended to avoid making visits to a doctor for minor issues like traditional cold and flu as the threat of contracting the coronavirus kept lurking around the corner.
All these developments translated into a considerable growth in the use of OTC products. Worthwhile benefits that people enjoyed were that it saved treatment costs incurred by paying a visit to hospitals and clinics. Thus with an increased focus on living a healthy lifestyle and enhancing body fitness, the demand for vitamins and mineral supplements continued to grow and is expected to grow further.
Bottom line
The pandemic has instilled waves of changes across many industries. As a result, the OTC industry has also come under its influence, unable to remain indifferent to the global changes. This has altered and changed many healthcare preferences and behaviors, with more and more people turning towards OTC products.
This implies that the OTC industry will need to adapt to the changes in the new normal. For this purpose, it needs to explore new ways of engaging its customers. This includes marketing their products to the customers both directly and indirectly. Possible actions they can take to boost their business and market share are investing and focusing more on educating the customers, undertaking regular reviews of product portfolios, and rethinking channel strategies to promote development. Among other critical steps that need to be taken include steps for enhancing marketing capabilities.
The OTC industry can benefit a lot by working closely with life sciences consulting companies. LST Consulting helps its clients on many platforms, including OTC products. We are in the prime position of providing a detailed OTC drugs market analysis which will allow our customers to focus on critical aspects that ensure success in the market, such as expert help on the distribution channels and product type. Our regional analysis, industrial insights, and expertise on product launch provide the catalyst to boost market share.
To learn more about the latest market trends and highlight the key industry dynamics related to OTC products, you may contact LST consulting services.

You must be logged in to post a comment.